Electronic submission website:
Contact person for the Joint Call Secretariat
TOPICS of the call:
Project proposals will address multidisciplinary and translational research. The project proposals must cover at least one of the following areas that are equal in relevance for this call:
General conditions for application:
A consortium applying to this call must include research group(s) from at least two out of the three following categories, if eligible according to relevant national/regional funding organisation’s regulations for research funding:
Please note! Whilst applications will be submitted jointly by groups from several countries, individual groups will be funded by the individual EuroNanoMed II funding organization respective of the country/region from which applicants have applied. The applications are therefore subjected to eligibility criteria of individual funding organizations. Please note that the national eligibility conditions have evolved since the last EuroNanoMed call. Applicants are therefore strongly advised to contact their national representative as soon as possible in order to confirm their eligibility with their respective funding organizations.
Contact Point list:
|
Party n° |
Participant organisation name |
Country/region |
Contact details |
|
P1 |
Agence Nationale de la Recherche (ANR) |
FRANCE |
Mariana Lassalle (+33) 1 73 54 81 93 Natalia Martin (+33) 1 73 54 81 33 |
|
P2 |
Agentschap voor Innovatie door Wetenschap en Technologie (IWT) |
BELGIUM / FLANDERS |
Dirk Veelaert: (+32) 2 432 42 19 |
|
P3 |
Service Public De Wallonie (SPW-DGO6) |
BELGIUM / WALLONIA |
nicolas.delsaux@spw.wallonie.be Nicolas Delsaux (+32) 81 33 45 20 |
|
P5 |
Vdi Technologiezentrum Gmbh (VDI) |
GERMANY |
Olaf Rotthaus (+49) 2 116214 – 233 |
|
P6 |
The Icelandic Centre For Research (RANNIS) |
ICELAND |
Katrín Valgeirsdóttir (+354) 8684743 |
|
P7 |
Chief Scientist Office, Ministry Of Health (CSO-MOH) |
ISRAEL |
Benny.Leshem@moh.health.gov.il Benny Leshem (+972) (0) 2 5681209 |
|
P8 |
Ministero Della Salute (IMH) |
ITALY |
Dr. Gaetano Guglielmi |
|
P11 |
Latvijas Zinatnu Akademija (LAS) |
LATVIA |
Yuri Dekhtyar (+371) 670 89 383 |
|
P12 |
Lietuvos mokslo taryba (RCL) |
LITHUANIA |
Julija Sabataityte (+370) 5 261 9068 |
|
P13 |
Norges Forskningsrad (RCN) |
NORWAY |
Cecilie Anita Mathiesen |
|
P14 |
Narodowe Centrum Badan i Rozwoju (NCBR) |
POLAND |
aleksandra.moscicka@ncbr.gov.pl Aleksandra Mościcka-Studzińska (+48) 22 39 07 131 |
|
P15 |
Fundação Para A Ciência E A Tecnologia (FCT) |
PORTUGAL |
Cristiana Leandro (+351) 213924 381 |
|
P17 |
Unitatea Executiva pentru Finantarea Invatamantului Superior, a Cercetarii, Dezvoltarii si Inovarii (UEFISCDI) |
ROMANIA |
Mihaela Manole (+40) 21 3023863 |
|
P18 |
Instituto De Salud Carlos III (ISCIII) |
SPAIN |
Ignacio Baanante (+34) 9182 22576 |
|
P19 |
Vetenskapsradet – Swedish Research Council(SRC) |
SWEDEN |
Johan Nilsson (+46) 8 546 44 202 |
|
P20 |
Schweizerischer Nationalfonds zur Förderung der wissenschaftlichen Forschung (SNSF) |
SWITZERLAND |
Christoph Meier (+41) 31 308 23 62 |
EuroNanoMed I & II call statistics
During the period 2009-2013, the ERA-NET EuroNanoMed I & II has successfully launched 4 joint calls for proposals.
The first 4 calls for proposals funded 31 transnational research projects on nanomedicine involving 165 partners from 21 countries/regions participating in the calls, with about € 32 million funding from the EuroNanoMed funding agencies and a further € 24 million funding from the research partners participating in the funded projects.
Country of origin of the applicants
Altogether, 648 partners from more than 21 countries participated in these calls, in a total of 141 submitted projects. As presented in figure 1, the number of applicants per country has globally grown between 2009 and 2013, as well as the number of submitted proposals (24 and 43 respectively).
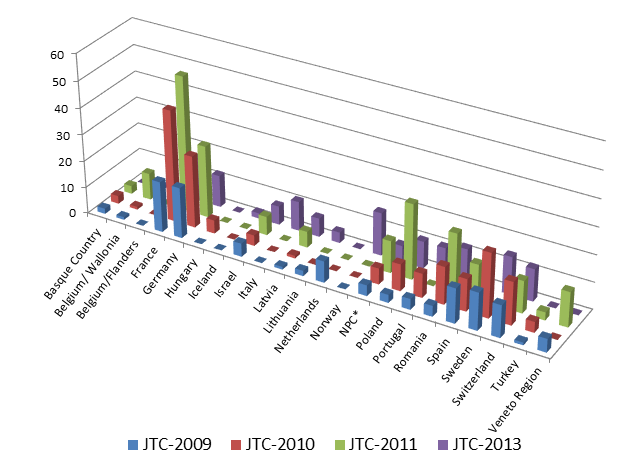 Figure 1: Number of applicants per country for the first 4 calls for proposals.
Figure 1: Number of applicants per country for the first 4 calls for proposals.
*NPC (Non participating country) refers to applicants that belonged to countries where no funding organization participated in the joint transnational call.
Overview of the 4 calls
The table below summarizes the outcomes of the first 4 calls for proposals:
Participation of research organizations in translational projects in Nanomedicine
As EuroNanoMed I & II joint calls for proposals aimed at funding translational research projects in Nanomedicine, each consortium had to include teams from at least 2 of the following categories: academia, clinic/public health, industry. The distribution between these three categories among consortia funded in the 4 calls is outlined in Figure 2. Noteworthy, 61% of the funded projects included partners from all three categories, indicating the high added value of these collaborations in nanomedicine translational research.
Figure 2: Type of research organizations funded through the four EuroNanoMed I & II joint calls for proposals.
Scientific / technological areas covered by funded projects
The first 4 calls for proposals were open to projects covering at least one of the three priority topics of the European Technology Platform Nanomedecine: Targeted delivery systems, Diagnostics, Regenerative medicine. The coverage of these three scientific areas among funded projects is presented in figure 3.
Figure 3: Distribution of scientific areas covered by funded projects in the first 4 calls for proposals.
Ⓒ All rights reserved EuroNanoMed III, 2018