The ERA-NET EuroNanoMed II launches a seventh Joint Call for Proposals to support transnational innovative research projects in Nanomedicine.
Research project consortia who intend to submit a transnational proposal should register from January 7th 2016, at https://www.pt-it.de/ptoutline/application/euronanomed2016, following the instructions provided on the site. Registration and electronic proposal submission must be completed before 11th February 2016 17:00:00 CET.
Contact details for the Joint Call Secretariat:
JCS is hosted by the French National Research (ANR)
Postal Address: 50 avenue Daumesnil, 75012 PARIS – France
Tel. +33(0)1 78 09 80 44
The aims of the call are:
Project proposals will address multidisciplinary and translational research. The project proposals must cover at least one of the following areas that are equal in relevance for this call:
A consortium applying to this call must include research group(s) from at least two out of the three following categories, if eligible according to relevant national/regional funding organisation’s regulations for research funding:
Please note! Whilst applications will be submitted jointly by groups from several countries, individual groups will be funded by the individual EuroNanoMed II funding organization respective of the country/region from which applicants have applied. The applications are therefore subjected to eligibility criteria of individual funding organizations. Please note that the national eligibility conditions have evolved since the last EuroNanoMed call. Applicants are therefore strongly advised to contact their national representative as soon as possible in order to confirm their eligibility with their respective funding organizations. Only transnational projects will be funded. Each proposal must involve a minimum of three research groups eligible for the funding organisations participating in the EuroNanoMed II 7th Joint Transnational Call. Moreover, these eligible research groups must come from at least three different countries and no more than two eligible research groups from the same country participating in the call will be accepted. Research groups not eligible to be funded (e.g. from non-funding countries or not fundable according to national/regional regulations of the participating funding countries) may participate in transnational projects if they are able to secure their own funding. However, the majority of research groups in a consortium and the coordinator must be eligible to be funded by EuroNanoMed II participating countries/regions. In any case, the maximum number of participants in a project consortium is seven.
|
Participant organisation name |
Country/region |
Contact details |
|
Agentschap voor Innovatie door Wetenschap en Technologie (IWT) |
BELGIUM / FLANDERS |
Geert Carchon (+32) 2 432 42 94 |
|
Fonds Wetenschappelijk Onderzoek (FWO) |
BELGIUM/ FLANDERS |
Olivier Boehme: (+32) 2 550 15 45 Toon Monbaliu (+32) 2 550 15 70 |
|
Fonds National de la Recherche Scientifique (FNRS) |
BELGIUM / BRUSSELS-WALLONIA |
Arnaud Goolaerts (+32 2 504 93 28) Freia Van Hee (+32 2 504 93 09) |
|
Service public de Wallonie (SPW-DGO6) |
BELGIUM / WALLONIA |
nicolas.delsaux@spw.wallonie.be Nicolas Delsaux (+32) 81 33 45 20 |
|
Agence Nationale de la Recherche (ANR) |
FRANCE |
Amélie Vergne (+33) 1 78 09 80 44 Guillaume Pons (+33) 1 73 54 83 32 |
|
Ministry of Education, Research and Religious Affairs (GSRT) |
GREECE |
Paraskevi Afentaki (+30) 210 7458112 |
|
Chief Scientist Office, Ministry Of Health (CSO-MOH) |
ISRAEL |
Ahmi Ben-Yehudah (+972) 2 5082163 Irit Allon (+972)2 5082167 |
|
Ministero Della Salute (IMH) |
ITALY |
Dr. Gaetano Guglielmi |
|
Latvijas Zinatnu Akademija (LAS) |
LATVIA |
Yuri Dekhtyar (+371) 670 89 383 Dace Tirzite (+371) 672 27 790 |
|
Lietuvos mokslo taryba (RCL) |
LITHUANIA |
Kornelija Janavičiūtė (+370) 5 210 7396 |
|
Norges Forskningsrad – The Research Council of Norway (RCN) |
NORWAY |
Cecilie Anita Mathiesen |
|
Fundação para a Ciência e a Tecnologia (FCT) |
PORTUGAL |
Carlos Almeida Pereira (+351) 213924 397 |
|
Unitatea Executiva pentru Finantarea Invatamantului Superior, a Cercetarii, Dezvoltarii si Inovarii (UEFISCDI) |
ROMANIA |
Mihaela Manole (+40) 21 3023863 |
|
Slovak Academy of Sciences (SAS) |
SLOVAKIA |
Katarina BIBOVA (+421) 2 5751 0136 Jan BARANCIK (+421) 2 5751 0137 |
|
Instituto de Salud Carlos III (ISCIII) |
SPAIN |
Dori Campo (+34) 9182 22489 |
|
Ministry of Economy and Competitiveness (MINECO) |
SPAIN |
Dr. Carles Cané (+34) 93 5947700 (+34) 916037269 |
During the period 2009-2016, the ERA-NET EuroNanoMed I & II have successfully launched 7 joint calls for proposals.
These joint calls for proposals funded 61 transnational research projects on nanomedicine involving 319 partners from 20 countries/regions participating in the calls and 5 additional non-participating countries, with about € 53.1 million funding from the EuroNanoMed funding agencies and a further € 36.7 million funding from the research partners participating in the funded projects.
Country of origin of the applicants
Altogether, 1659 partners from 32 countries participated in these joint calls, in a total of 326 submitted projects. As presented in figure 1, the number of applicants per country has globally grown between 2009 and 2016, as well as the number of submitted proposals (from 24 to 68 respectively).
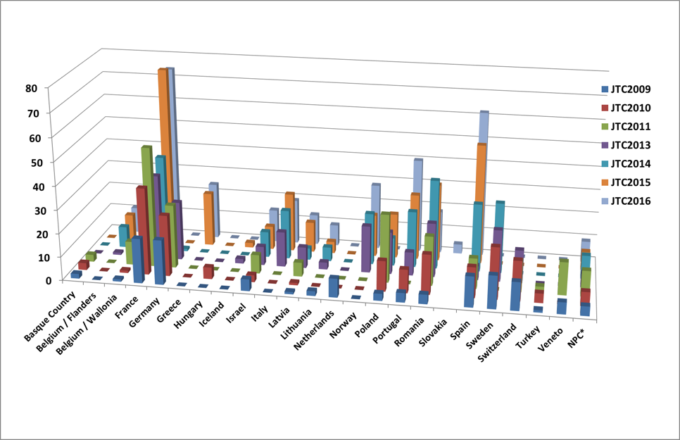
Figure 1: Number of applicants per country for the 7 joint calls for proposals.
*NPC (Non-Participating Country) refers to applicants from countries that did not participate in the joint transnational call.
Overview of the 7 joint calls
The table below summarizes the outcomes of the 7 joint calls for proposals:
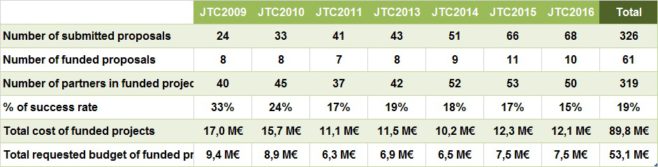
Participation of research organizations in translational projects in Nanomedicine
As EuroNanoMed I & II joint calls for proposals aimed at funding translational research projects in Nanomedicine, each consortium had to include teams from at least 2 of the following categories: academia, clinic/public health, industry. The distribution between these three categories among consortia funded in the 7 joint calls is outlined in Figure 2. Noteworthy, 53% of the funded projects included partners from all three categories, indicating the high added value of these collaborations in nanomedicine translational research.
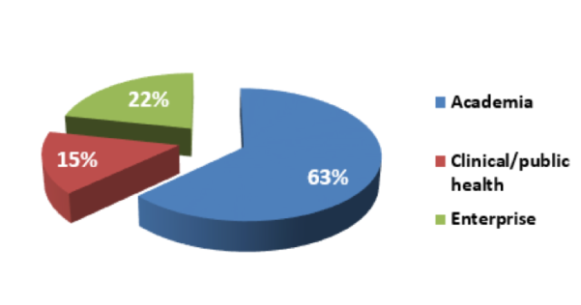
Figure 2: Type of research organizations funded through the 7 joint calls for proposals.
Scientific / technological areas covered by funded projects
The 7 joint calls for proposals were open to projects covering at least one of the three priority topics of the European Technology Platform Nanomedicine: Targeted delivery systems, Diagnostics, Regenerative medicine. The coverage of these three scientific areas among funded projects is presented in figure 3.

Figure 3: Distribution of scientific areas covered by funded projects in the 7 joint calls for proposals.
Ⓒ All rights reserved EuroNanoMed III, 2018